The anticonvulsant drug ezogabine, (also known as retigabine), which opens the KCNQ2/3 potassium channel in the brain, has been found to improve depression and anhedonia—a lack of reactivity to pleasurable stimuli—in the first randomized, placebo-controlled,
proof-of-concept trial of its kind. Results from the trial, led by researchers at the Icahn School of Medicine at Mount Sinai, were published in the March 3, 2021 issue of the American Journal of Psychiatry and provide important evidence that the KCNQ2/3 channel could serve as a new target for drug discovery.
Ezogabine was approved by the U.S. Food and Drug Administration in 2011 as an anticonvulsant for epilepsy treatment but had not been previously studied in depression.
“Targeting this channel represents a completely different mechanism of action than any
currently available antidepressant treatment,” says James Murrough, MD, PhD, Associate Professor of Psychiatry, and Neuroscience, Director of the Depression and Anxiety Center for
Discovery and Treatment at Icahn Mount Sinai, and senior author of the paper.
The KCNQ2/3 channel is a member of a large family of ion channels referred to as the KCNQ (or Kv7) family that act as important controllers of brain cell excitability and function in the central nervous system. These channels affect brain cell function by controlling the flow of the electrical charge across the cell membrane in the form of potassium (K+) ions.
“Given that more than one-third of people suffering from depression are inadequately treated with currently approved therapeutics, new treatments are urgently needed.”
James Murrough, MD, PhD
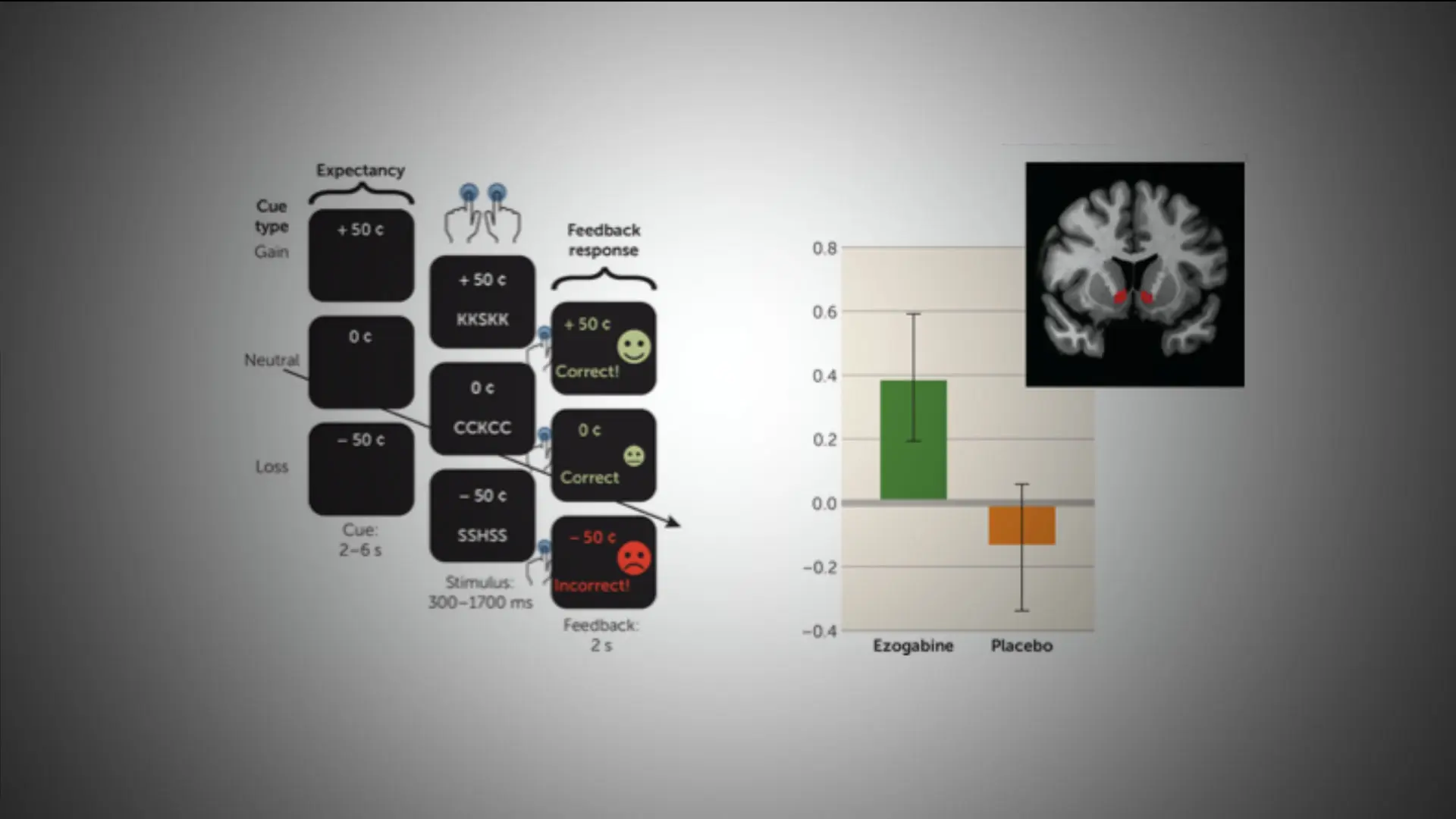
In the current double-blind trial, 45 adult patients diagnosed with a depressive disorder were assigned to a five-week treatment period with daily dosing of either ezogabine or matching placebo. All participants underwent clinical evaluations and functional magnetic resonance imaging.
Those treated with ezogabine showed a significant reduction in several key measures of depression severity, anhedonia, and overall illness severity. Significant improvements following treatment with ezogabine compared to placebo were also observed.
Study co-author, Ming-Hu Han, PhD, Professor of Pharmacological Sciences, and Neuroscience, at Icahn Mount Sinai, had previously conducted a series of studies showing that mice that appeared to be resistant to developing depression when stressed showed an increase in the function of the KCNQ2/3 channel in the brain. Dr. Han’s laboratory also
discovered that if depressed mice were given a drug such as ezogabine—which increased the activity of this channel—they no longer showed depressive and anhedonic behaviors because the drug acted as an antidepressant.
“The fundamental insight by Dr. Han’s group that a drug that essentially mimicked a mechanism of stress resilience in the brain could represent a whole new approach to the treatment of depression was very exciting to us,” says Dr. Murrough. “Given that more
than one-third of people suffering from depression are inadequately treated with currently approved therapeutics, new treatments are urgently needed.”
*Study authors James Murrough, MD, PhD, and Ming-Hu-Han, PhD, are named inventors on a pending patent application for the use of ezogabine and other KCNQ channel openers to treat
depression and related disorders.
Featured

James Murrough, MD, PhD,
Director of the Depression and Anxiety Center

Ming-Hu Han, PhD,
Professor of Pharmacological Sciences, and Neuroscience